'Pulse charging' can double the battery life of smartphones and laptops

Many people who use smartphones and laptops for a long time are troubled by the problem that the batteries wear out over time and run out quickly even when fully charged. Research results have been announced that it is possible to double the lifespan of lithium-ion batteries used in many devices by charging them using a '
Unravelling the Mechanism of Pulse Current Charging for Enhancing the Stability of Commercial LiNi0.5Mn0.3Co0.2O2/Graphite Lithium‐Ion Batteries - Guo - Advanced Energy Materials - Wiley Online Library
https://onlinelibrary.wiley.com/doi/10.1002/aenm.202400190
Tired of your laptop battery degrading? New 'pulse current' charging process could double its lifespan. | Live Science
https://www.livescience.com/technology/electronics/tired-of-your-laptop-battery-degrading-new-pulse-current-charging-process-could-double-its-lifespan
BESSY II - How pulsed charging enhances the service time of batteries - Batteries News
https://batteriesnews.com/bessy-ii-how-pulsed-charging-enhances-the-service-time-of-batteries/
Lithium-ion batteries, which are found in many products from smartphones to electric cars, deteriorate as they undergo hundreds of charging cycles (each cycle consisting of one charge and discharge), gradually losing their maximum capacity.
According to a research team from Humboldt University Berlin in Germany and Aalborg University in Denmark, the most advanced lithium-ion batteries today use lithium nickel manganese cobalt oxide (NMC532) and graphite electrodes and have a lifespan of about 5 to 8 years, which translates into 300 to 500 charging cycles.

Previous research has suggested that a more advanced charging protocol, called pulsed current, could extend the lifespan of these lithium-ion batteries, but the mechanism behind this was not well understood, preventing practical application.
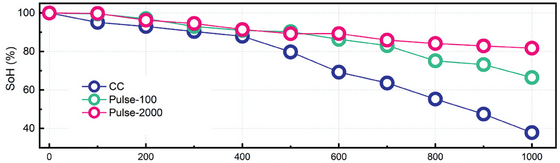
To investigate the effect of different charging methods on battery degradation, the research team conducted experiments in which commercially available lithium-ion batteries were charged with constant and pulsed currents and the health of the batteries was diagnosed every 100 cycles. As a result, it was confirmed that the battery life was doubled when charged with pulsed current.
The graph below summarizes the results of the experiment. The battery charged with constant current (CC) reached its effective end of life after 500 cycles, with its charge capacity falling below 80%, and after 1000 cycles only 37.8% remained. On the other hand, the battery charged with a pulse current (Pulse-100) with a frequency of 100Hz lasted for 700 cycles before reaching 80%, and maintained 66.48% of its capacity even after 1000 cycles. And the battery charged with Pulse-2000 with a frequency of 2000Hz maintained more than 80% of its performance even after 1000 cycles.
When the research team analyzed lithium-ion batteries that had been repeatedly charged and discharged, they found that in batteries charged at a constant current, a membrane called the solid electrolyte interface (SEI) formed on the surface of
In addition, the electrodes made of NMC532 and graphite also had numerous cracks, which also contributed to the loss of capacity. In contrast, the batteries charged with pulsed current had a thinner SEI and suffered less damage to the electrodes.
The following are microscopic images of the surface of an electrode (Graphite), from left to right: a new electrode (Fresh), a constant current (CC), and a 2000Hz pulse current (Pulse-2000). The electrode charged with a constant current (center) had a film of about 110nm, but with the pulse current (right), this was reduced to about half, about 50nm.

'These findings provide insights into optimizing charging methods for current lithium-ion batteries to extend their service life and further develop future battery technologies,' the research team wrote in a paper published in the journal Advanced Materials Sciences on March 14, 2024.
Related Posts: