The insulin administration app for diabetes patients 't:connect', which has injured 224 people due to a sudden app shutdown, is being recalled

On March 26, 2024, California-based medical device manufacturer
Tandem Diabetes Care, Inc. Recalls Version 2.7 of the Apple iOS t:connect Mobile App Used in Conjunction with t:slim X2 Insulin Pump with Control-IQ Technology Prompted by a Software Problem Leading to Pump Battery Depletion | FDA
https://www.fda.gov/medical-devices/medical-device-recalls/tandem-diabetes-care-inc-recalls-version-27-apple-ios-tconnect-mobile-app-used-conjunction-tslim-x2

FDA recalls iOS app software that injured over 200 insulin pump users - The Verge
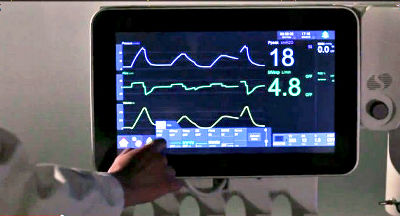
The t:slim X2 insulin pump is a device that delivers insulin subcutaneously to diabetes patients. When used with t:connect and other devices, it can adjust insulin delivery based on current glucose measurements and predicted therapy, and administer additional insulin as needed. t:connect can also display insulin pump information on devices such as smartphones and limit pump control.
However, since version 2.7, there have been reports of the iOS version of t:connect crashing and restarting repeatedly, as well as issues with pump batteries draining rapidly due to excessive Bluetooth communication. A rapid battery drain can cause the pump to shut down prematurely, causing insulin delivery to stop, which can be fatal for severe diabetes patients.
Tandem Diabetes Care points out that 'hyperglycemia that becomes severe due to prolonged lack of insulin administration can lead to

According to the FDA, 224 diabetes patients have been reported to have been injured by the t:connect app malfunction. As of April 15, 2024, no deaths related to this issue have been reported.
Nevertheless, the FDA has classified the t:connect app as a Class 1 recall, the most serious category, for product issues that could lead to serious illness or death. In response, Tandem Diabetes Care recalled version 2.7 of t:connect on March 26, 2024, and sent an Emergency Medical Device Correction Letter to all affected users.
The number of devices subject to the recall is 85,863, and Tandem Diabetes Care is informing t:connect users to update the app to version 2.7.1 or later, which fixes the problem, as soon as possible.
Related Posts: