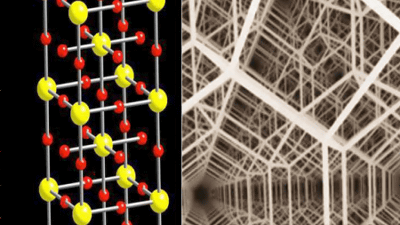
Technology developed to produce synthetic diamonds at normal pressure

Natural
Growth of diamond in liquid metal at 1 atm pressure | Nature
https://www.nature.com/articles/s41586-024-07339-7

Making diamonds at ambient pressure | EurekAlert!
https://www.eurekalert.org/news-releases/1042017
Forget Billions of Years: Scientists Have Grown Diamonds in Just 150 Minutes : ScienceAlert
https://www.sciencealert.com/forget-billions-of-years-scientists-have-grown-diamonds-in-just-150-minutes
In the HPHT method, which is used to produce 99% of synthetic diamonds, diamonds are generally grown at temperatures ranging from 1300 to 1600 degrees Celsius, with pressures of 5 to 6 GPa and liquid metal as a catalyst. Synthetic diamonds are already used in a wide range of fields, such as jewelry and precision machining tools, but if a method can be found to produce synthetic diamonds at lower pressures and in a shorter time, it could revolutionize the production of synthetic diamonds.
To speed up the process of finding new ways to create synthetic diamonds, the Korean research team developed a homemade vacuum system called the RSR-S, which significantly accelerated their research into adjusting parameters such as the ratio of liquid metals and temperature, and finally discovered the parameters that could produce synthetic diamonds under normal pressure.
The method of producing synthetic diamonds discovered by the research team is to expose pure methane and hydrogen to liquid metal mixed with gallium, nickel, iron and silicon in a ratio of '77.75: 11.00: 11.00: 0.25' under conditions of 1 atmosphere (about 1013 hPa) and 1025 degrees Celsius. The carbon atoms contained in methane spread under the surface of the liquid metal, small diamond nuclei were formed in just about 15 minutes, and finally a diamond film was formed on the surface of the liquid metal, the research team reported.
Yan GONG, a graduate student at Ulsan Institute of Science and Technology and lead author of the paper, said, 'One day, I was using the RSR-S system to conduct an experiment, cooling a graphite crucible to solidify the liquid metal, and removing the solidified liquid metal pieces. I noticed that a rainbow-like pattern spread over several millimeters on the bottom surface of the pieces. It turned out that this rainbow color was caused by diamond! This allowed us to identify parameters that are favorable for reproducible growth of diamonds.'

The researchers also found that the subsurface region of the liquid metal in contact with the synthetic diamonds was an amorphous region 30-40 mm thick, with about 27% of the atoms in the upper part of this region being carbon atoms. Carbon is thought to be insoluble in gallium, so it was unexpected that such a high concentration of carbon could be found in a gallium-rich liquid metal.
They also found that the small amount of silicon present in the liquid metal played an important role in the growth of synthetic diamonds. When the amount of silicon was increased beyond the optimum, the diamonds were smaller in size and denser, whereas without silicon, the diamonds could not grow at all. This suggests that silicon may be involved in the initial formation of synthetic diamonds.
In this study, a liquid metal consisting of gallium, nickel, iron and silicon was used, but it seems that it was possible to grow high-quality diamonds even if the nickel was replaced with cobalt or the gallium was replaced with a mixture of gallium and indium.
'Our findings about diamond nucleation and growth in liquid metals are fascinating and offer many exciting opportunities for fundamental science,' said Rodney Ruoff, director of the Institute for Basic Science's Center for Multidimensional Carbon Materials. 'We are now exploring when nucleation and the subsequent rapid growth of diamond occur.'
Related Posts:
in Science, , Posted by log1h_ik